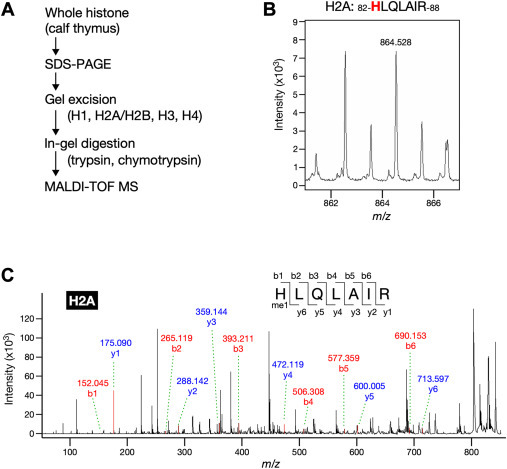
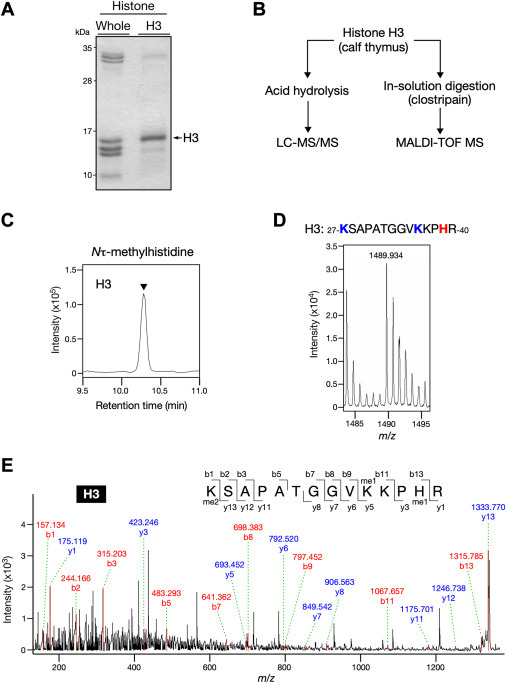
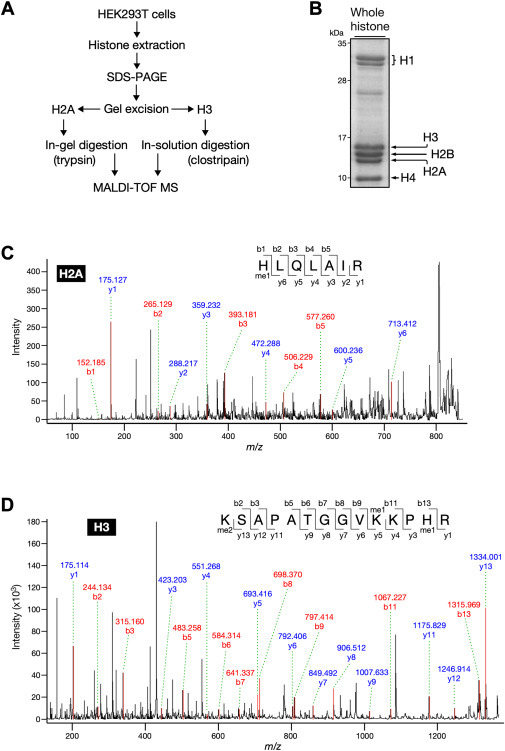
・We set out to determine whether histidine methylation occurs in histone using a unique method of analyzing methyl amino acids derived from hydrolysate of histone proteins combined with liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS).
・MALDI-TOF MS analysis revealed that histones H2A and H3 were methylated at His-82 and His-39, respectively.
・Our findings provided evidence that histones indeed carry a τMH modification and suggest that this methylation has additional implications for the histone code hypothesis.
Abstract
In contrast to the methylation on lysine and arginine, histone histidine methylation has not yet been firmly established. We aimed to investigate the occurrence of histidine methylation in histone proteins using highly sensitive mass spectrometry. We found that acid hydrolysates of whole histone fraction from calf thymus contained Nτ-methylhistidine, but not Nπ-methylhistidine. Both core and linker histones carried a Nτ-methylhistidine modification, and methylation levels were relatively high in histone H3. Furthermore, through MALDI-TOF MS, we identified two histidine methylation sites at His-82 in the structured globular domain of histone H2A and His-39 in the N-terminal tail of histones H3. Importantly, these histidine methylation signals were also detected in histones purified from a human cell line HEK293T. Moreover, we revealed the overall methylation status of histone H3, suggesting that methylation is enriched primarily at lysine residues and to a lesser extent at arginine and histidine residues.
Benefit
The combination of modern MS/MS with highly purified histones has revealed that histidine Nτ-methylation occurs, at least in histones H2AH82 and H3H39. Our findings established histidine Nτ-methylation as a new histone modification, which may serve as a chemical flag that mediates the epigenetic mark of adjacent residues of the N-terminal tail and the conformational properties of the globular domain.
Market Application
A unique method of analyzing methyl amino acids derived from hydrolysate of histone proteins combined with liquid chromatography coupled to tandem mass spectrometry to determine whether histidine methylation occurs in histone.
Publications
https://www.sciencedirect.com/science/article/pii/S0021925823021592?via%3Dihub